Polystyrene Structure
Polystyrene is a type of vinyl polymer. It consists of a lengthy hydrocarbon chain where every second carbon atom is connected to a phenyl group. It’s manufactured through the free radical polymerization process using the styrene monomer.
Polystyrene is a thermoplastic polymer derived from the styrene monomer and is naturally transparent. It is commercially available in two forms, namely solid plastic and rigid foam material.
One of the distinctive features of polystyrene is that it softens upon heating, and its films and sheets can be molded into various shapes for use in different applications.
It is one of the largest plastics commodities globally, holding approximately 7% of the market share in the thermoplastic market.
In this article, I will give a detailed outlook on polystyrene structure and how it affects the polymer’s general properties and other aspects.
Chemical Structure and Composition of Polystyrene
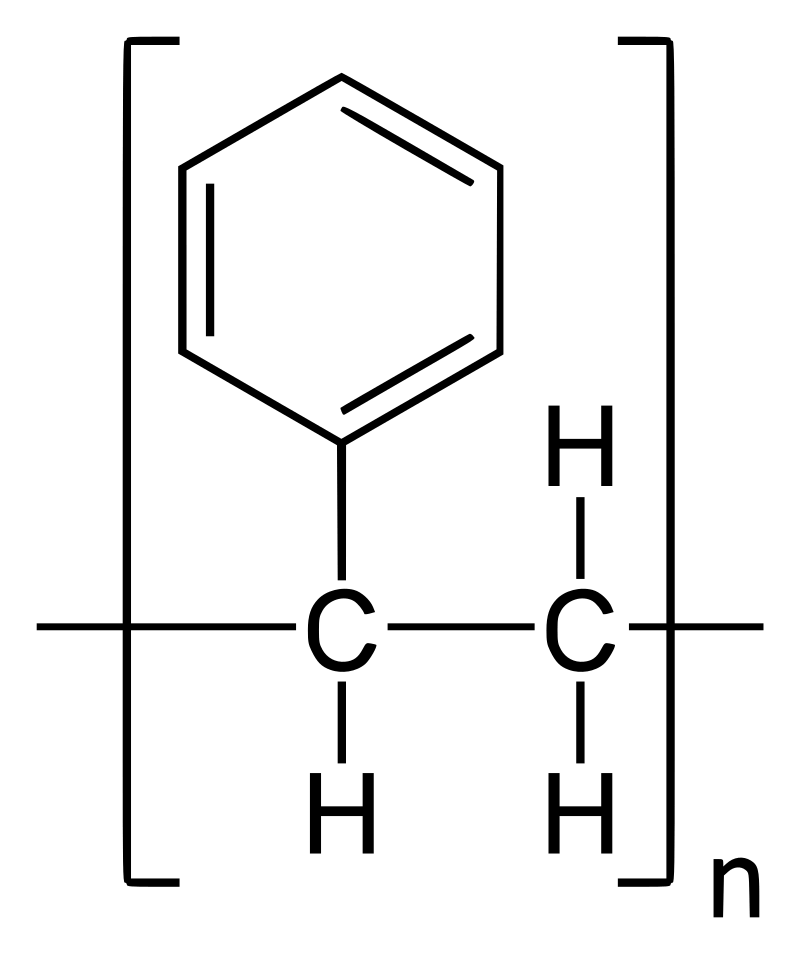
Polystyrene is a linear polymer comprising a recurring unit of styrene monomers, an organic compound with the molecular formula C8H8.
The polymerization of styrene monomers occurs via addition polymerization, wherein the double bond present in the styrene molecule is cleaved, and covalent bonds link together the resultant monomers to form a lengthy chain-like polymer.
The chemical composition of polystyrene can be denoted as [CH2-CH(C6H5)]n, where n is the number of repeating units.
The repeat unit of the polystyrene polymer is the styrene monomer, which comprises a vinyl group and a benzene ring.
The benzene ring is a six-membered carbon ring with alternating single and double bonds, while the vinyl group is composed of two carbon atoms bonded by a double bond and attached to a hydrogen atom.
The chemical structure of polystyrene gives it unique physical and mechanical properties, such as transparency, hardness, and density.
The molecular weight of polystyrene can vary from a few thousand to several hundred thousand, depending on the production process.
Different methods are available for producing polystyrene, such as suspension polymerization, emulsion polymerization, and mass polymerization.
These methods vary in how they disperse the styrene monomers in the reaction medium and the conditions under which the polymerization process occurs.
The selection of the production method can influence the mechanical and physical properties of the polystyrene product.
Molecular Weight of Polystyrene
The molecular weight of polystyrene is dependent on the number of styrene monomers that have undergone polymerization.
Polystyrene can have varying molecular weights, ranging from several thousand to millions of Daltons.
Polystyrene’s molecular weight can be determined through several techniques, such as gel permeation chromatography (GPC) and mass spectrometry.
The molecular weight of polystyrene is a crucial factor that influences its properties.
Polystyrene with high molecular weight exhibits increased rigidity and a higher melting point when compared to low molecular weight polystyrene.
Moreover, as the molecular weight of polystyrene increases, its viscosity also increases, leading to difficulties in processing.
Polystyrene Structure’s Role in Its Applications
Polystyrne’s rigid structure is crucial in applications requiring shape retention and mechanical strength, such as producing disposable cutlery, CD cases, and laboratory equipment.
Moreover, polystyrene’s capacity to resist moisture and chemicals further expands its utility in these areas.
Another notable aspect of polystyrene is its capacity for thermal insulation.
Expanded Polystyrene (EPS), a variant, contains many tiny air pockets within its matrix.
These air pockets significantly reduce thermal conductivity, making EPS an ideal material for insulation panels in building construction and temperature-sensitive packaging in the food and pharmaceutical industries.
Moreover, the ease of molding and shaping polystyrene is integral to its widespread use in packaging and consumer products.
Its lightweight nature, combined with structural integrity, ensures efficient transport and protection of goods while reducing shipping cost
How Polystyrene Structure Affects Its Processing
Polystyrene structure is a crucial factor in determining its processing properties. The molecular weight of the polymer is one of the main factors influencing processing.
A higher molecular weight increases viscosity, which can pose challenges during processing, especially in melting processes like injection molding and extrusion.
This higher viscosity can result in slower processing speeds and increased energy consumption.
Therefore, it is essential to consider the molecular weight of polystyrene during processing to avoid difficulties and ensure efficient production.
Polystyrene’s rigidity is another structural feature that can affect its processing.
Its relatively inflexible structure makes it more prone to brittleness, which can cause processing challenges such as cracking and breaking.
Plasticizers can be added as additives to mitigate this issue to enhance flexibility.
The method used to produce polystyrene can notably impact its processing properties.
The production method can affect the distribution of molecular weight, which, in turn, can impact the material’s properties.
Therefore, it is essential to select the appropriate production method to determine the processing properties of polystyrene.
Summary
The Molecular structure of polystyrene makes it a widely used polymer in various industries due to its low cost, easy processability, and lightweight.
Polystyrene has a unique combination of physical and chemical properties, making it suitable for packaging, insulation, and other applications.
However, the environmental impact of polystyrene is a concern due to its non-biodegradable nature and potential harm to wildlife.
Therefore, it is essential to consider sustainable alternatives and proper disposal methods to mitigate its negative environmental impact.
Thanks for reading. Have a wonderful day.
Quick Navigation