Nylon, also known as Polyamide, is a linear thermoplastic material that is highly durable and strong, making it a popular choice for engineering applications. It has exceptional tensile strength and is often used as a substitute for silk, rubber, and latex materials.
Nylon Structure
Nylon is widely used in various applications, including textiles, rubber components like tires, ropes, threads, automobile parts, and mechanical components.
An American chemist by the name of Wallace Carothers is credited with the development and initial testing of Polyamide or Nylon in 1935. The specific type of nylon produced by Carothers was Nylon 66, which remains the most widely used variant to this day.
In this article, I will give a detailed outlook on the structure of Nylon and how it affects the polymer’s general properties and other aspects.
Chemical Structure and Composition of Nylon
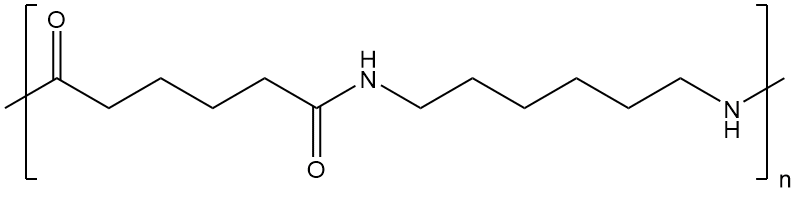
Nylon’s chemical composition is defined by recurring amide groups resulting from the reaction between a dicarboxylic acid and a diamine.
Adipic acid is the most commonly used dicarboxylic acid in nylon manufacturing, while hexamethylenediamine is the most frequently utilized diamine.
To produce nylon-6,6, adipic acid and hexamethylenediamine undergo a chemical reaction, as demonstrated by the following equation:
HOOC(CH2)4COOH + H2N(CH2)6NH2 → HOOC(CH2)4CO-NH-(CH2)6-NH-CO(CH2)4COOH + H2O
The resulting product is a polyamide, which consists of long chains of repeating units of amide groups.
The number 6,6 in nylon-6,6 refers to the number of carbon atoms in the diamine and dicarboxylic acid, respectively. The reaction between caprolactam and water produces nylon-6.
Molecular Structure of Nylon
Nylon’s molecular structure is distinguished by lengthy chains of repeating units of amide groups. These amide groups are made up of a carbonyl group (C=O) and an amino group (NH2) linked by a single bond (-NH-CO-).
The resulting structure forms a linear polymer with robust intermolecular forces between adjacent chains.
The length of its polymer chains determines the molecular weight of nylon and can differ based on this factor. Nylon-6,6 has a molecular weight of approximately 226 g/mol, whereas nylon-6 has a molecular weight of roughly 113 g/mol.
The molecular weight of nylon has an influence on its characteristics, with higher molecular weights typically resulting in greater strength and durability.
Nylon can also exist in different forms, including crystalline and amorphous. Crystalline nylon is characterized by its ordered molecular structure, while amorphous nylon has a disordered molecular structure.
The degree of crystallinity can also impact the properties of nylon, with higher degrees of crystallinity leading to increased stiffness and strength.
How Structure of Nylon Affects Its Properties?
The Nylon structure is crucial in determining its properties, such as melting point and physical, thermal, and mechanical properties.
The amide groups in the chemical structure of nylon create a polarity level that contributes to its relatively high melting point.
In addition, the strength of the intermolecular forces between nylon chains, which is determined by the number and strength of hydrogen bonds, also affects its melting point.
Therefore, nylon melting point can vary based on factors such as its molecular weight and the degree of crystallinity in the polymer.
For example, Nylon-6,6 usually has a higher melting point than Nylon-6.
The physical properties of nylon, including its tensile strength and elasticity, are significantly impacted by its structure.
The long chains of amide groups create a high degree of molecular alignment, leading to strong intermolecular forces between adjacent chains.
This molecular alignment results in excellent tensile strength, making nylon desirable for applications requiring high-strength materials, such as ropes, threads, and mechanical components.
Additionally, the presence of polar amide groups provides a degree of moisture absorption, which can affect the material’s dimensional stability.
Nylon’s thermal properties are heavily impacted by its chemical structure. The presence of polar amide groups results in relatively high dielectric strength, making nylon an excellent electrical insulator.
Additionally, nylon has a low coefficient of friction and remarkable wear resistance, making it an ideal material for gears and bearings.
The polar amide groups and the degree of crystallinity in the polymer also influence nylon’s thermal stability. Overall, nylon displays good thermal stability, with a melting point typically exceeding 200°C.
The chemical structure of nylon significantly impacts its mechanical properties. The long, linear chains of amide groups give nylon high tensile strength and excellent abrasion resistance.
Its exceptional elasticity and toughness make it an ideal material for applications that require a material with greater density capable of enduring repeated loading, such as ropes and threads.
Nylon’s toughness is also influenced by its degree of crystallinity and molecular weight, with increased degrees of crystallinity and molecular weight generally leading to improved toughness.
Summary
To sum up, the nylon structure, which is unique and distinctive, has contributed to its broad and versatile use as a polymer. Its remarkable durability, flexibility, and chemical resistance have rendered it an invaluable material for various applications, from clothing and carpets to automotive components. Nylon’s fundamental repeating unit comprises two monomers that can be altered to produce multiple forms of nylon with distinct properties.
Understanding nylon’s chemical structure and its effects on its properties is crucial for producing customized materials that cater to the distinct needs of diverse industries.
Quick Navigation
