Polymerization techniques can differ from each other because there are multiple polymerization methods with different purposes and results. One of them is step growth polymerization vs chain growth polymerization.
Step Growth VS Chain Growth Polymerization
Step growth and chain growth are extensive methods of polymerization. They utilize monomers with distinct attributes and characteristics and display different growth patterns.
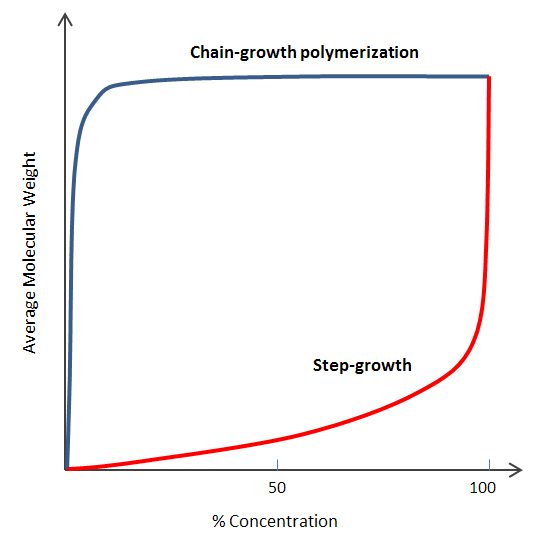
In the chain growth process, the polymer chain extends incrementally, adding one monomer at each stage. Conversely, in step growth, the polymer chain size doubles with each successive step. Therefore, these two methods present significantly different growth rates for the polymer chain. Chain growth, specifically, involves a consistent expansion in chain length with each addition phase.
Condensation polymerization is a step-growth polymerization where molecules join, losing small molecules as by-products, such as water and methanol. It produces linear polymers from bifunctional monomers. i.e., compounds with two reactive end groups. The polymerization takes place between larger structural units and monomers. The condensation growth steps are expressed by:
Px+Py→Px+y+L {x}∈{1,2,…∞};{y}∈{1,2,…∞}
The most common condensation polymers are polyamides, bakelite, starch, polyacetal, polycarbonates, kevlar, epoxies, and polyurethanes. Polyamide or Nylon 6-6 is produced via a condensation reaction between 1,6-hexanedioic acid (or adipic acid) and 1,6-hexanediamine (or hexamethylenediamine). A proton is lost by the amine end group of the nucleophile, and an OH group is lost by carboxylic acid.
On the other hand, anionic polymerization is a form of chain-growth polymerization that involves monomers initiated with anions of vinyl monomers with strong electromagnetic groups. It is mainly utilized to produce synthetic polydiene rubbers, thermoplastic styrene elastomers, and solution styrene-butadiene rubbers (SBR).
It starts with a nucleophilic attack on a monomer, creating a carbanion. Fundamentally, all vinyl monomers with strong electronegative replace one or more atoms in the parent chain of hydrocarbons (also called substituents) in the presence of these anions.
Certain electron-replacing substituents have the ability to counterbalance the negative charge using charge delocalization, making the anionic polymerization steady and smoother in nature.
The key difference in both chain growth vs step growth polymerization is that in chain growth, the polymer chain always grows one monomer at a time. On the other hand, in step growth, the polymer chain doubles with each step. That’s why the rate of growth of the polymer chain is drastically different in these two cases.
The Difference Between Step Growth vs Chain Growth Polymerization
Source - Polymerdatabase.com
| Step Growth Polymerization | Chain Growth Polymerization |
| All the monomers, oligomers, and polymers can react with any other molecule. |
During dispersion, monomers only react to the active site at the end of the growing chain.
|
| There is no termination step at the end group of polymers and oligomers, which are reactive throughout the polymerization process. |
There are two very different mechanisms during polymerization – Initiation, and propagation. In most instances, there is also a termination step.
|
| Monomers are present throughout the reaction, but many monomers are consumed very early in the reaction. |
Monomers are present throughout the reaction, but the overall number decreases steadily during the reaction.
|
| The reaction is processed rapidly at the start, but the increase in the molecular weight is slow. High molecular weight is only achieved at the end of the process by long oligomers reacting with each other. |
The reaction speed depends on the concentration of the initiator and co-initiator, and a high molecular weight polymer is created throughout the time span of the reaction.
|
| Oligomers are present throughout the reaction, with length distribution shifting to higher molecular weight with increasing reaction time. |
The mixture contains mostly monomers and polymers and only little amounts of growing polymer chains.
|
| Long reaction times are a must for the fusion of long and high molecular weight polymers. |
Long reaction times have degrees of metamorphosis but do not affect the molecular weight.
|
Difference Between Step Growth and Chain Growth Polymerization
FAQs
Below are the frequently asked questions about the difference between chain growth and step growth polymerization. Let’s dig deep to learn more.
Is PVC a step-growth polymer?
Yes, PVC, along with mainstream polymers like polyethylene (PE), polypropylene (PP), polymethyl methacrylate, polyacrylonitrile, and polyvinyl acetate, are classified as step-growth polymers.
What is the difference between chain-growth and step-growth polymers?
A chain-growth polymer always grows one monomer at a time. However, in step growth, the polymer chain doubles with every step. As a result, the rate of growth in both polymers is drastically different from each other.
What are the 4 types of polymers?
Polymers are categorized into 4 types – thermoplastics, thermosets, elastomers, and synthetic fibers. It should be noted that synthetic polymers are man-made polymers.
What is the definition of polymerization?
A process in which relatively small molecules, called monomers, join chemically to produce a giant chainlike molecule called polymer is known as polymerization.
What are the steps of polymerization?
Any polymerization reaction consists of three steps – Initiation, Propagation, and termination.
Suggested Read
- What is HDPE Material? | HDPE Properties | Advantages of HDPE | Disadvantages of HDPE | The Future of HDPE
- What is LDPE Material? | The Complete Guide
- What is UHMW Plastic Material? | The Definitive Guide
- What is Acrylic Plastic? | The Ultimate Guide
- What is Acetal? | Acetal Copolymer and Homopolymer | Acetal Applications | Advantages & Disadvantages of Acetal
- How to Choose the Right Lubricants For Plastic Parts?
The Takeaway
In conclusion, step growth vs chain growth offers distinct mechanisms for polymer chain development, each impacting the rate of growth and final polymer properties differently. Understanding these processes is crucial in tailoring materials for specific applications. Chain growth provides a steady, step-by-step approach, while step growth expedites the process, doubling chain length at each stage. Choosing the appropriate polymerization strategy is a vital aspect of advanced materials science.
Please feel free to leave your feedback and ideas in the comment section.
Wishing you an incredible day.
Quick Navigation