The making process of plastic is attributed to be complicated. It’s expensive and goes through multiple procedures to reach its final stage. Let’s get into the details and try to understand how plastic is made.
How is Plastic Made?
Plastic is made from base resources such as natural gas, oil, or plants. These resources undergo refining processes to produce ethane and propane. Subsequently, ethane and propane are subjected to a heat treatment in a procedure known as “cracking,” transforming them into ethylene and propylene. These components are then amalgamated to form diverse polymers.
There are mainly two types of plastics – 1) Synthetic and 2) Bio-based. Synthetic plastics are extracted from petrol products like crude oil, natural gas, or coal.
Conversely, bio-based plastics are derived from renewable products like vegetable fats and oils, starch, corn, carbohydrates, bacteria, etc.
Synthetic plastics dominate the plastic processing industry because of their ease of processability.
However, the shortage of oil reserves worldwide has led to the slow adoption of bio-plastics extracted from animal waste and biomass.
Plastics are made utilizing a total of four steps. Here are they:
- Extracting the raw materials
- Refining Process
- Polymerization
- Compounding
Extraction of Raw Materials
It all begins with extracting raw materials from crude oil, natural gas, and sometimes coal. The overall mixture needs to be processed under particular conditions. Handling the variety is a complex task as it contains thousands of compounds.
Refining Process
The transformation of crude oil and natural gases into various petroleum products is called the refining process. The derived petroleum products are converted into valuable chemicals, including “monomers.”
Crude oil is heated in a furnace and sent to the distillation unit. Here the filtration will occur, separating crude into lighter and smaller compounds called “fractions.”
The plastic industry favors a range of hydrocarbon distillates out of the extracted compounds, collectively called Naptha. It can make large amounts of plastic, but other means, like gas, can also be utilized.
Polymerization
Most plastics are produced using Polymerization (Chain Growth Method) and the step-growth method.
Polymerization begins with olefin gas converting into higher molecular weight hydrocarbons(Polymers). To make that possible, monomers must be bonded into chains. There are two variations of polymerization – Addition Polymerization and Condensation Polymerization.
Addition Polymerization
By its name, addition polymerization means one monomer connecting to another(dimer), relating to another(trimer), and so on. To achieve this perfectly, a catalyst named Peroxide should be used.
Common materials derived from addition polymerization are Polystyrene (PS), Polyvinyl chloride (PVC), and Polyethylene (PE).
Condensation Polymerization
It means combining multiple monomers by removing small molecules like water. A catalyst is also necessary to make a reaction between adjacent monomers. Adding an existing chain to another is quite common to get desired results.
Typical examples are polyester and nylon.
Compounding
Compounding means mixing various blends within a specific temperature to get formulations of plastics. The mixing is carried out using an extruder, fastening the mixture.
After that, the pallets are brought to the shop floor, where they are processed in injection molding or any other processing method to transform them into finished or semi-finished products.
The processing nature is completely dependent on the end application. It can vary in size, shape, color, and properties.
Engaging Read – What is the Density of Plastics? | The Complete Guide
Identification of Plastics
We just learned how plastic is made. Now, let’s take a look at the classification of plastics. The classification depends on many factors like the reaction to chemicals, melting point, applications, and properties.
- Thermoplastics and Thermosets
- Amorphous and semi-crystalline
- Homopolymers and Copolymers
| Thermoplastics | Thermosets |
| It can be heated multiple times without significant degradation, which can be injected, molded easily, and later recycled. |
It can only be heated once during the processing cycle as heating changes its chemical nature into 2-part epoxy. Continuous heating will burn the material.
|
| The overall usage in the plastic processing industry is more than in thermosets. |
Overall usage is less because of its chemical nature and is utilized in particular applications.
|
| The molecular structure involves a series of repeat units combined linearly. |
The molecular structure involves a two-three dimensional design instead of one-linear in thermoplastics.
|
| Great candidates for recycling |
Poor candidates for recycling
|
| Common examples are poltypropylene(PP), acrylonitrile-butadlene-styrene, Polyoxymethylene(POM) |
Typical examples are Polyurethane(PUR), phenolic, epoxy, silicone, etc.
|
The classification between amorphous and semi-crystalline materials is dependent on their molecular structure.
| Amorphous materials |
Semi-crystalline materials
|
| Amorphous material lacks long-range symmetry like semi-crystalline material and thus becomes soft when additional heat is applied. |
Semi-crystalline materials have a more defined structure and exhibit a smooth transition from solid to liquid using a small temperature range.
|
| Applications – Tyres, hoses, gaskets, seals, etc |
Applications – Bearings, gears, structural loads, etc
|
| Examples – acrylonitrile-butadiene-styrene(ABS), Polystyrene(PS), Polycarbonate(PC), polysulfone |
Examples – Polyethylene(PE), polyethylene terephthalate (PET), Polypropylene(PP)
|
Talking homopolymer and copolymer, their classification is based on the final material’s monomer makeup. A monomer is a molecule that combines with other molecules to become a larger molecule (polymer).
| Homopolymer | Copolymer |
| If the final plastic is made up of a single type of monomer, it is called a homopolymer. |
If plastic is made from two or more different types of monomers, then it is called a copolymer.
|
| Homopolymers have greater room temperature impact strength. |
Copolymers have better dimensional stability and tensile strength.
|
| Homopolymers have greater room temperature and impact strength. |
Copolymer possesses better chemical resistance.e
|
| Examples – are polypropylene, Polymethyl-methacrylate, etc. |
Examples – are polyethylene-vinyl acetate (PEVA), acrylonitrile butadiene styrene (ABS), etc.,
|
Bonus:- Let’s get into more details about what is plastic made from.
What are Hydrocarbons?

Hydrocarbons are a class of organic compounds, which can be either aliphatic or aromatic. Aliphatic hydrocarbons do not contain benzene rings, while aromatic hydrocarbons do. Carbon, represented as C with an atomic number of 6, possesses a valency of four, which means it has four electrons in its outermost shell.
These electrons can bond with electrons from any other element in the periodic table. For hydrocarbons, carbon pairs up with hydrogen. Hydrogen symbolized as H and having an atomic number of 1, possesses only one electron in its outer shell, which means that four hydrogen atoms can form a bond with a single carbon atom to create a CH4 molecule, also known as methane.
Methane is the simplest hydrocarbon and the first member of the Alkane series. Two carbon atoms can also bond together, pairing with six hydrogen atoms to form a CH3-CH3 molecule, also known as ethane. This pattern continues in the Alkane series.
The Alkane series includes methane (CH4), ethane (C2H6 or CH3-CH3), propane (CH3-CH2-CH3), butane (CH3-CH2-CH2-CH3), pentane (CH3-CH2-CH2-CH2-CH3), and continues with hexane, heptane, octane, nonane, dodecane, undecane, and so forth.
3In this case, the bonds formed between carbon and hydrogen are saturated, also known as sigma bonds (σ-bonds). However, unsaturated bonds can also exist, featuring a pi bond (π-bond) alongside a sigma bond.
These bonds form double bonds between carbon atoms (alkenes) or triple bonds (alkynes), depending on the hybridization between the elements.
The Alkene series includes ethylene (C2H4 or CH2=CH2), propylene (CH2=CH-CH3), 1-butylene (CH2=CH-CH2-CH3), 2-butylene (CH3-CH=CH-CH3), and so forth. It’s worth noting that 1-butylene and 2-butylene are isomers of butylene.
How is Synthetic Plastic Created From Crude Oil?
Synthetic plastic is derived from petrochemicals. When an oil deposit is located beneath the Earth’s surface, drilling operations are conducted through the rocky strata to access and extract the oil.
Oil extraction involves pumping the oil from deep underground to the surface, loaded onto tankers for transportation to shore. Offshore drilling operations also occur, supported by platforms situated in the ocean. Depending on the size of the pumps, anywhere from 5 to 40 liters of oil can be produced per pump stroke.
The refining process involves transferring the oil through pipelines, which can span thousands of miles, to an oil refining facility. If oil spills occur during this transportation process, they can pose immediate and long-term environmental threats. However, various safety protocols are implemented to avoid and reduce the risk of such spills.
Crude oil distillation and petrochemical production – Crude oil, a complex mixture of hundreds of hydrocarbons, includes some solids and gaseous hydrocarbons, mostly alkanes such as CH4 and C2H6, but sometimes also C3H8 or C4H10.
Initially, the crude oil is heated in a furnace, channeling the resultant vapor mixture into a fractional distillation tower. This tower divides the mixture into several segments, referred to as fractions.
A temperature gradient exists within the distillation tower, with the top being cooler than the bottom. The liquid and vapor fractions separate within the tower according to their weight and boiling point (the temperature at which a liquid transforms into a gas).
When evaporating vapors encounter a liquid fraction whose temperature is lower than the vapor’s boiling point, they partially condense.
These evaporating crude oil vapors condense at different temperatures within the tower. Lighter fraction vapors (gasoline and petroleum gas) ascend to the top of the tower, mid-weight liquid fractions (kerosene and diesel oil distillates) occupy the middle, and heavier liquids (referred to as gas oils) segregate towards the bottom. In contrast, the heaviest fractions (solids) with the highest boiling points remain at the base.
Each fraction in the column consists of hydrocarbons with a similar count of carbon atoms, with smaller molecules towards the top and longer molecules closer to the bottom.
Thus, petroleum is broken down into petroleum gas, gasoline, paraffin (kerosene), naphtha, light oil, heavy oil, etc.
What is the Difference Between Plastic and Polymer?
The terms ‘polymer’ and ‘monomer’ originate from Greek, with ‘poly’ meaning ‘many’, ‘mer’ signifying ‘repeating unit’, and ‘mono’ translating to ‘one’. In essence, a polymer comprises numerous repeating monomer units. Plastics vs Polymers is a term which confuses people even today
Polymers are larger molecules that are formed when many monomer units are covalently bonded together, much like beads strung together on a string. The term ‘plastic’ derives from ‘plasticus’ in Latin and ‘plastikos’ in Greek, referring to something ‘capable of molding’ or ‘fit for molding’.
Hence, when we mention plastics, we typically refer to organic polymers, both synthetic and natural, of high molecular weight that are combined with other substances. Plastics are high molecular-weight organic polymers containing carbon, hydrogen, oxygen, nitrogen, sulfur, and chlorine elements.
They can also be synthesized from a silicon atom (resulting in silicone) along with carbon; a well-known example includes silicone breast implants or silicone hydrogel used for optical lenses.
Plastics are primarily composed of polymeric resin, often blended with other substances called additives. ‘Plasticity’ is a term denoting the characteristic or trait of a material that allows it to deform irreversibly without breaking.
It indicates whether a polymer can withstand the temperature and pressure conditions during the molding process.
Chemistry provides the capacity to adjust various parameters to modify the properties of polymers. By using different elements, altering the types of monomers, and rearranging them in diverse patterns, we can change the shape of the polymer, its molecular weight, or other chemical or physical properties.
This adaptability allows plastics to be tailored to possess the necessary properties for specific applications.
How is Plastic Made From Naphtha?

Naphtha is a range of distillate hydrocarbons responsible for the creation of plastics. It is a combination of C5 to C10 Hydrocarbons.
Naphtha is kept in a steam cracker in hot water and decomposed at a temperature (~800 °C), divided into two major hydrocarbons known as major intermediaries.
These small molecules are joined together, making a long chain called a polymer. When making their way to the shop floor, these polymers are shaped like granules (sometimes powder when processing in rotational molding ).
Before they take the shape of our everyday, beautiful-looking plastic products, they go through intense heating, melting, and cooling processes within different processing methods (Injection Molding, Extrusion, Blow molding, etc.)
The Future of Plastics
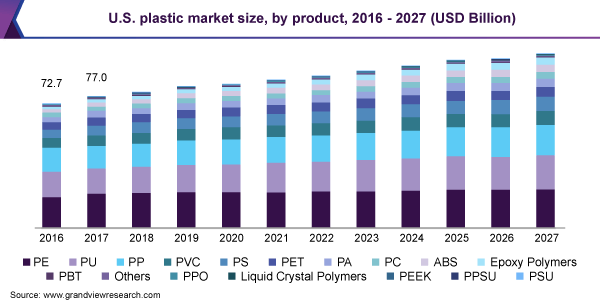
Shoutout – Grandviewresearch.com
According to serval research conducted by Grand View Research, the global plastics market is valued at USD 568.9 billion in 2019 and will register a CAGR of 3.2% for 2020-2027. Packaging, construction, electronics, and electrical are the largest plastic consumers in business verticals.
Consumer Goods, medical, and Agriculture are also growing rapidly and can hold a significant chunk of the future market share for plastic consumption.
Global Plastic Market Share, By Industry (2019)
However, the recent outbreak of Covid-19 has hindered the growth of plastics and overall manufacturing, construction, and other verticals because of nationwide lockdowns, supply chain constraints, and no economic activity whatsoever.
As of working on this piece, a vaccine rollout is on the cards, and with government stimulus spending worldwide, things can get better in no time. So the long-term growth outlook is positive.
FAQs
Below are the frequently asked questions on how is plastic produced and what is plastic made of. Let’s dig deep to know more.
Who made the first plastic?
One of the earliest examples of the invention of plastics was in 1855 by Alexender Parkes, and he nomenclature his invention Parkesine, which we today know as celluloid. After that, a breakthrough came with the creation of Polyvinyl chloride somewhere between 1838-1872.
A quantum leap in the plastic invention story came in 1907 when Belgian-American scientist Leo Baekeland invented Bakelite, the first synthetic mass-produced plastic (still being used but meagrely).
What are the 7 types of plastics?
Below are the seven most common types of plastics:
1) Polyethylene Terephthalate (PET or PETE)
2) High-Density Polyethylene (HDPE)
3) Polyvinyl Chloride (PVC or Vinyl)
4) Low-Density Polyethylene (LDPE)
5) Polypropylene (PP)
6) Polystyrene (PS or Styrofoam)
7) Others (ABS, Polycarbonate, Biodegradable plastic, etc.
Which is the safest plastic to drink from?
There are multiple plastic materials used in consumer applications. However, according to my knowledge and experience, the best plastic to drink or consume anything from is HDPE. Most detergent and juice bottles, milk jugs, toiletries containers, butter tubs, and even a good amount of water bottles (Although the most utilized plastic for making water bottles is PET with more than 70% market share) are also made from HDPE.
What are the steps of the plastic recycling process?
There are six main steps in the plastic recycling process. Here are they:
1. Collection of waste plastic
2. Organizing plastics into categories
3. Washing to remove impurities
4. Stressing and resizing
5. Identification and uncoupling of plastics
6. Compounding
What are the most common plastic processing methods?
The most common plastic processing techniques are Injection molding, Extrusion, rotational molding, blow molding, thermoforming, and compression molding.
Suggested Read
- What is the Density of Plastics? | The Complete Guide
- Top 5 Heat Resistant Plastic Materials | A List of High Temp Plastic Materials
- 6 Best Plastic Molding Techniques | A Complete Analysis
- What is Liquid Plastic? | Liquid Plastic Vs. Resin | An In-Depth Guide
Final Words
I have tried to keep this piece short. So that was my take on how are plastics made. The plastic creation process is complicated and takes much time and investment to even start. However, innovation has been made to make the process more transparent and inexpensive and less harmful to the environment. So keep researching this and stay updated for the best knowledge.
Kindly share your reviews in the comment box.
Quick Navigation


How will oil on PVC parts regrined into pellets effect remelt for a second injection molding cycle
That was a good read. I appreciate the writer’s efforts to mention the details on naphtha distillation
Thanks.