Polyethylene terephthalate (PET) is a polymer comprising recurring units of terephthalic acid and ethylene glycol. Its unique structure provides durability and chemical resistance, making PET a widely-used material in textiles and packaging, with notable recyclability potential.
PET Structure
PET plastic, also called PETE, is a widely used thermoplastic material that finds its applications in textiles, electronics, packaging, automobiles, and films globally.
The textile industry predominantly utilizes PET, commonly known as Polyester.
PET exhibits impressive properties, such as chemical resistance, dimensional stability, and mechanical and thermal resistance, making it highly desirable to plastic manufacturers.
This article will provide an in-depth analysis of the structure of PET and its impact on the polymer’s general properties and other relevant factors.
Chemical Composition and Molecular Unit of PET
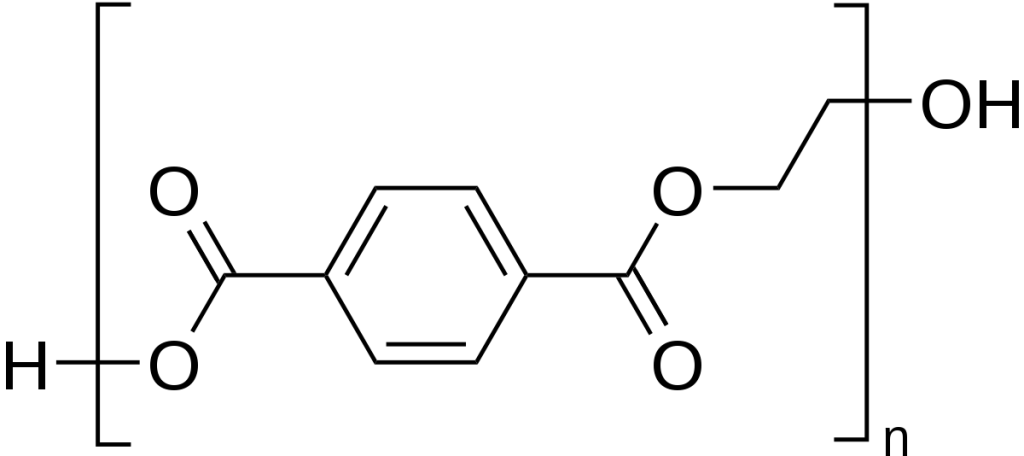
Its chemical composition largely determines the properties and applications of PET.
PET is a polymer comprising repeating ethylene terephthalate units formed by condensing terephthalic acid and ethylene glycol.
This synthesis process yields a linear polyester polymer that can be processed into diverse forms.
PET’s repeating unit, ethylene terephthalate, comprises two distinct components: ethylene glycol and terephthalic acid.
The former contributes to the polymer’s flexibility and softness, while the latter imparts strength and rigidity.
Its molecular weight significantly influences PET’s properties.
The synthesis process can regulate the molecular weight of PET by adjusting reaction conditions like pressure, temperature, and catalysts.
Higher molecular weight PET typically exhibits greater strength and rigidity, while lower molecular weight PET tends to be more flexible and more accessible to process.
PET’s resistance to various chemicals is primarily determined by its chemical composition.
PET exhibits high resistance to water, most acids and bases, and many solvents, making it a preferred choice for packaging materials that require durability against harsh chemicals.
Nevertheless, PET is not impervious to some substances, including alkaline solid solutions and certain organic solvents.
Crystalline Structure
PET possesses a crystalline structure that emerges from the alignment of polymer chains.
This structure confers PET’s strength, rigidity, and resistance to deformation under stress.
The manufacturing process, including factors such as temperature and pressure, influences PET’s crystalline structure.
PET exhibits a semi-crystalline nature, implying that the polymer chain comprises crystalline and amorphous regions.
The crystalline domains of PET are meticulously arranged with a repeating structure, while the amorphous regions lack any ordered arrangement.
By regulating the processing conditions, the proportion of crystalline and amorphous regions in PET can be adjusted, thereby enabling the customization of the material’s properties to suit specific applications.
How PET Chemical Structure Affects its Properties
Various aspects related to PET plastic are deeply affected by its chemical structure. Let’s learn more about the most critical elements.
PET’s melting point is mainly determined by the degree of crystallinity in the polymer.
The highly ordered and tightly packed crystalline regions have a higher melting point than the disordered amorphous regions.
Therefore, PET with a higher degree of crystallinity will have a higher melting point. The melting point of PET typically ranges between 240-260°C.
The mechanical properties of PET, such as strength, density, and flexibility, are heavily influenced by its degree of crystallinity.
PET with a higher degree of crystallinity tends to be stronger, stiffer, and more rigid, while PET with a lower degree of crystallinity is more flexible and has a lower modulus of elasticity.
The processing conditions during manufacturing can also be adjusted to modify the mechanical properties of PET.
The physical properties of PET, such as its density, transparency, and gas barrier properties, are closely linked to its crystalline structure.
PET with a high degree of crystallinity typically has a higher density, greater transparency, and better gas barrier properties than PET with a lower degree of crystallinity.
This is because the highly ordered crystalline regions provide a more tightly packed molecular structure, which leads to higher density and improved gas barrier properties.
Additionally, the regular arrangement of molecules in the crystalline regions allows for more excellent light transmission, resulting in higher transparency.
The physical properties of PET can also be influenced by the processing conditions used during manufacturing.
The crystalline structure of PET also affects its thermal properties, including its glass transition temperature and heat resistance.
PET with a higher degree of crystallinity has a higher glass transition temperature, indicating better heat resistance than PET with a lower degree of crystallinity.
Moreover, incorporating additives during processing can further enhance PET’s heat resistance.
Summary
To sum up, the properties and applications of PET are highly influenced by its structure, which consists of repeating units of ethylene terephthalate.
The degree of crystallinity in PET affects its melting point and mechanical, physical, and thermal properties.
PET with a high degree of crystallinity possesses better thermal properties, rigidity, and strength.
Moreover, its physical attributes like density, transparency, and gas barrier properties depend on the crystalline structure.
Properly comprehending PET structure is necessary to optimize and tailor its properties for diverse industrial and consumer applications.
Thanks for reading. Kindly share your reviews and questions in the comment box.
Quick Navigation